The question of whether the surface roughness of a zirconia dental implant determines its ultimate ability to integrate into the bone has been at the center of the scientific debate surrounding ceramic implants for quite some time. Studies suggest that zirconia implants with smooth endosseous surfaces are associated to comparatively long healing periods.[1,2, 5, 6] This is in line with findings from Cionca et al., who argue that the surface of the endosseous portion of a zirconia implant should be as rough as possible to achieve reliable osseointegration.[5]
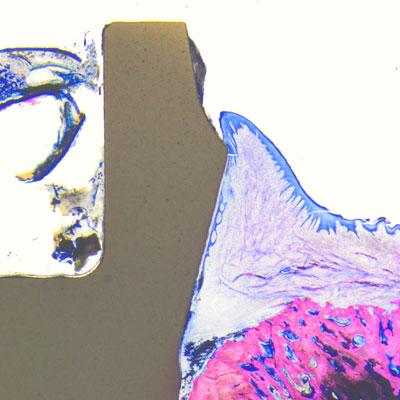
Histology samples were analyzed using Leica light microscopes and bone-to-implant contact (BIC) was measured from the most crestal bone-implant contact point to the apex of the implant (© Peter Schüpbach).
In order to evaluate whether a high endosseous surface roughness leads to accelerated and reliable osseointegration of zirconia implants, Swiss researchers Dr. Roland Glauser and Dr. Peter Schüpbach conducted an experimental pre-clinical animal study with the aim of examining the early bone formation and bone healing mechanisms around two-piece Patent™ zirconia implants with a modified rough endosseous surface.6 They found that the Patent™ Implant, thanks to its highly rough BBS surface, outperforms all other dental implants in terms of bone healing speed and success that have been investigated in comparable studies to date. The findings of their study are now officially published in the International Journal of Implant Dentistry under the title “Early bone formation around immediately placed two-piece tissue-level zirconia implants with a modified surface: an experimental study in the miniature pig mandible” (accessible at: https://doi.org/10.1186/s40729-022-00437-z ).
Scope
Six premolars were extracted in four miniature pigs (species sus scrofa domesticus). Five zirconia soft-tissue-level implants (Patent™ Standard Two-piece Implant; 4.1 mm in diameter and 11 mm in length; Zircon Medical Management AG, Switzerland) and one titanium tissue-level control implant (Straumann® Standard RN Roxolid; 4.1 mm in diameter and 10 mm in length; SLActive® surface) were immediately placed in the extraction sockets of each animal under full sedation. The Patent™ Implants investigated are produced from Yttria-stabilized zirconia in a patented manufacturing process. This process involves the sand-blasting of the endosseous implant surface in the pre-sintering stage. The resulting BBS surface (“blasted before sintering”) has a roughness of Ra 5.7 µm in its endosseous portion. This especially rough surface is approximately five times rougher than other documented zirconia implants.[7, 8] Its transmucosal portion is machined. The titanium control implants have an endosseous surface roughness of Ra 2.2 µm, and a machined transmucosal portion. After implant placement, the flaps were carefully closed with sutures and the implants were left for transmucosal healing. No levelling of the bone was performed and no grafting procedures or membranes were used. No implants were lost during the healing phase. Two animals were sacrificed after four weeks and two after eight weeks. Histology samples were analyzed using Leica light microscopes. Bone-to-implant contact (BIC) was measured from the most crestal bone-implant contact point to the apex of the implant.
Key take-aways
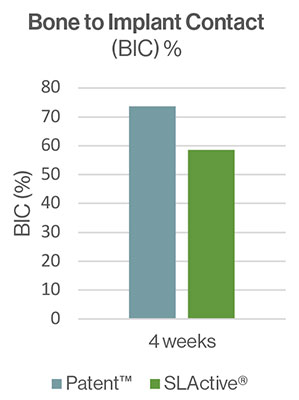
The Patent™ test implants demonstrated high rates of osseointegration four and eight weeks after insertion. After four weeks the BIC reported for the test implant was 73,7% (SD ± 16,8) and 58,5% for the control. After eight weeks of healing, both implant types were completely osseointegrated with BIC of 82.4% (SD ± 16.9) and 93.6% (SD ± 9.1), respectively. Contact osteogenesis was observed directly on and along the surface of the Patent™ Implants. The authors conclude that the surface of the Patent™ Implant investigated may be classified as highly osteoconductive. They also state that the presence of bone debris is of vital importance to accelerate the initial bone formation process.[9] During implant placement, a rough implant surface scratches off bone along the walls of the osteotomy. This creates a micron-thick smear layer consisting of bone debris and blood, covering part of the implant surface immediately following installation.[10, 11] The bone debris guides new bone formation by distance osteogenesis to the implant surface. The authors suggest that the rougher surface of the Patent™ test implants may have generated more osteogenic bone debris and smear layer compared to the control implants, contributing to the high BIC ratio at four weeks of healing.
Conclusion
Based on their findings from the experimental pre-clinical study, authors Dr. Glauser and Dr. Schüpbach conclude that the endosseous surface roughness of zirconia dental implants has a decisive influence on their ability to integrate into the surrounding bone. They report that the immediately placed two-piece Patent™ zirconia implants achieve fast and predictable osseointegration thanks to their highly rough endosseous BBS surface. The results of this animal study demonstrate a higher mean BIC ratio for the Patent™ Implant investigated compared with previous studies evaluating surface-modified zirconia implants in similar animal models.
References
1. Akagawa Y, Ichikawa Y, Nikai H, Tsuru H. Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J Prosthet Dent. 1993;69(6):599–604.
2. Scarano A, Di Carlo F, Quaranta M, Piatelli A. Bone response to zirconia ceramic implants: an experimental study in rabbits. J Oral Implantol. 2003;29(1):8–12.
3. Kohal RJ, Weng D, Bachle M, Strub JR. Loaded custom-made zirconia and titanium implants show similar osseointegration: an animal experiment. J Periodontol. 2004;75(9):1262–8.
4. Sennerby L, Dasmah A, Larsson B, Iverhed M. Bone tissue responses to surface-modified zirconia implants: a histomorphometric and removal torque study in the rabbit. Clin Implant Dent Relat Res. 2005;7(s1):s13–20.
5. Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol. 2017;73(1):241–58.
6. Glauser, R., Schupbach, P. Early bone formation around immediately placed two-piece tissue-level zirconia implants with a modified surface: an experimental study in the miniature pig mandible. Int J Implant Dent 8, 37 (2022). https://doi.org/10.1186/s40729-022-00437-z
7. Oliva J, Oliva X, Oliva JD. One-year follow-up of first consecutive 100 Zirconia dental implants in humans: a comparison of 2 different rough surfaces. Int J Oral Maxillofac Implants. 2007;22(3):430–5.
8. Roehling S, Schlegel KA, Woelfler H, Gahlert M. Zirconia compared to titanium dental implants in preclinical studies—a systematic review and meta-analysis. Clin Oral Implants Res. 2019;30(5):365–95.
9. Bosshardt DD, Salvi GE, Huynh-Ba G, Ivanovski S, Donos N, Lang NP. The role of bone debris in early healing adjacent to hydrophilic and hydrophobic implant surfaces in man. Clin Oral Implants Res. 2011;22(4):357–64.
10. Dhore CR, Snel SJ, Jacques SV, Naert IE, Walboomers XF, Jansen JA. In vitro osteogenic potential of bone debris resulting from placement of titanium screw-type implants. Clin Oral Implants Res. 2008;19(6):606–11.
11. Tabassum A, Walboomers F, Wolke JG, Meijer GJ, Jansen JA. Influence of the surgical technique and surface roughness on the primary stability of an implant in artificial bone with a density equivalent to the maxillary bone: a laboratory study. Clin Implant Dent Relat Res. 2011;13(4):269–78.





