In his landmark 2012 review examining the influence of different implant designs and materials on soft- and hard-tissue stability, Belgian researcher Prof. Eric Rompen defined the specific characteristics that a dental implant system must possess in order to achieve long-term treatment success and guarantee the preservation of stable marginal bone levels.1 The Patent™ Dental Implant System successfully incorporates all the characteristics necessary for long-term implant treatment success, according to Prof. Rompen.
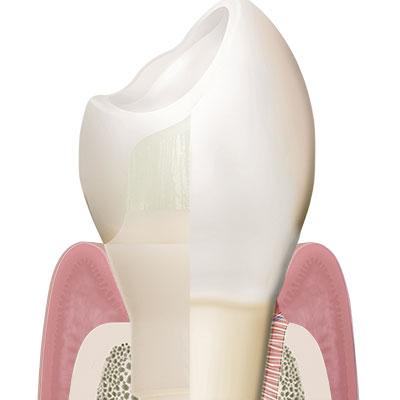
Patent™ has a soft-tissue-level design that positions the crown margin at the equigingival level.
Among other things, Rompen argues that tissue-level designs are advantageous over bone-level designs with bacteria-prone microgaps at the subgingival level. Having a soft-tissue-level design, the Patent™ Implant neither incorporates a microgap at the subgingival level nor at the level of the crestal bone. Its crown seat and the implant platform, in which the glass fiber core build-up is cemented, are instead positioned at the equigingival level. As a result, the crown margin of the final restoration is easily accessible and easy to clean, which greatly reduces the risk of bacteria-induced inflammation of the peri-implant soft- and hard tissues.
Designed for long-term oral health
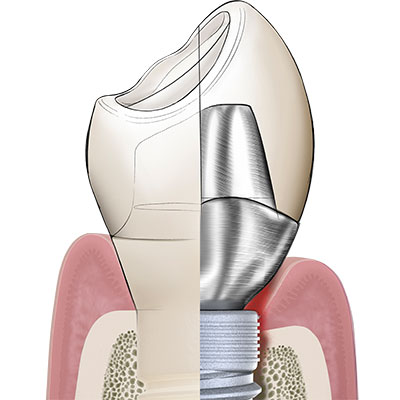
In its endosseous portion the Patent™ Implant has a threaded part with an ideal surface roughness of approximately Ra 5,7 µm that extends into a threadless (“thread-off”) part above it. A 2.5 mm tall tulip-shaped transgingival collar rises from the implant body. This portion of the implant is machined and does not undergo any surface treatment after milling to allow optimum soft-tissue adhesion. From this transgingival tulip emerges a partial abutment (the ferrule) which incorporates the 3.3 mm deep 3C™ connection at its center.
The ferrule effect
Dentists worldwide are familiar with the concept of a ferrule within the context of endodontic treatment: In order to provide an endodontically treated tooth with fracture resistance and its crown with stability, a portion of the upper part of the tooth is prepared into a ferrule, in which the post and core are inserted. The integrated partial abutment of the two-piece Patent™ Implant is the equivalent of a ferrule on an endodontically treated tooth. The ferrule of the Patent™ Implant is typically supragingival, because it is always incorporated inside the body of the crown. The ferrule incorporates the 3C implant connection at its center, in which the glass fiber post is cemented. Once cemented, the post is prepared using a high-speed diamond drill – just like any post would be prepared in endodontic dentistry. The glass fiber post, having dentinlike properties, is able to attenuate the masticatory forces within the context of the definitive restoration in a favorable way. Once cemented and prepared to form, the post is restored with a crown or any other kind of definitive prosthesis. Once could say that Patent™ is a one-piece implant which gives you the flexibility of a two-piece implant.to them.

The correct implant position
If the Patent™ Implant is placed correctly according to the manufacturer’s surgical protocol (and not inserted too deeply), the interface between the crown margin and the implant table is at the equigingival level and the ferrule with the 3C implant connection at its center is at the supragingival level. Without there being a bacteria-prone subgingival microgap, the peri-implant soft tissues can begin to adhere firmly to the transgingival tulip in the form of a cuff shortly after insertion, largely free of irritation and undisturbed by bacterial pathogens. Because of this so-called “early sealing” of the mucosa to the transgingival implant portion, the risk of subgingival cement residues or glass fiber components (as a result of the intraoral cementation process) is virtually non-existent.to them.
Proven by science
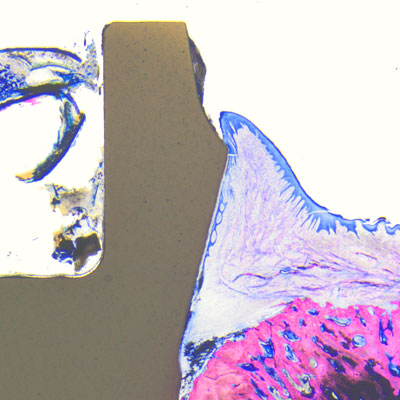
Thanks to its soft-tissue-level design, among other things, the Patent™ Implants does not incorporate a subgingival microgap susceptible to the infiltration and accumulation of bacterial pathogens, which would promote the development of peri-implant inflammation and marginal bone loss. As a result, the stability and the health of the peri-implant hard soft and hard tissues is maintained over the long term. This has been scientifically proved: Becker et al. and Brüll et al. observed an increase in keratinized gingiva around inserted Patent™ Implants in their 2- and 3-year clinical studies after the respective study periods.2, 3 In the long-term follow-up study by Becker et al. Patent™ Implants placed in patients with average health profiles showed healthy soft-tissue conditions with mucosal recession of <1 mm, stable bone and soft-tissue levels and no signs of periimplantitis—even after almost a decade of wear.4 Also, as part of a retrospective long-term study, Dr. Sofia Karapataki observed over 90 Patent™ Implants inserted in a cohort of compromised patients for a functional period of up to 12 years and she found that none of the implants investigated showed signs of peri-implantitis.5 to them.
References:
1. Rompen E. The impact of the type and configuration of abutments and their (repeated) removal on the attachment level and marginal bone. Eur J Oral Implantol. 2012;5 Suppl:S83-90. PMID: 22834397
2. Becker J, John G, Becker K, Mainusch S, Diedrichs G, Schwarz F. Clinical performance of two-piece zirconium implants in the posterior mandible and maxilla: a prospective cohort study over 2 years. Clin. Oral Impl. Res. 28, 2017, 29–35. doi: 10.1111/clr.12610
3. Brüll F, van Winkelhoff AJ, Cune MS. Zirconia dental implants: a clinical, radiographic, and microbiologic evaluation up to 3 years. Int J Oral Maxillofac Implants. 2014 Jul-Aug;29(4):914-20. doi: 10.11607/jomi.3293
4. Rauch N, et al. 2022. Two-piece zirconia implants in posterior regions: a prospective cohort study with a follow-up period of 9 years.
5. Karapataki S, Fahrenholtz H, et al. Peri-implantitis and zirconia implants: results after five and up to 12 years of function. In preparation.
1. Rompen E. The impact of the type and configuration of abutments and their (repeated) removal on the attachment level and marginal bone. Eur J Oral Implantol. 2012;5 Suppl:S83-90. PMID: 22834397
2. Becker J, John G, Becker K, Mainusch S, Diedrichs G, Schwarz F. Clinical performance of two-piece zirconium implants in the posterior mandible and maxilla: a prospective cohort study over 2 years. Clin. Oral Impl. Res. 28, 2017, 29–35. doi: 10.1111/clr.12610
3. Brüll F, van Winkelhoff AJ, Cune MS. Zirconia dental implants: a clinical, radiographic, and microbiologic evaluation up to 3 years. Int J Oral Maxillofac Implants. 2014 Jul-Aug;29(4):914-20. doi: 10.11607/jomi.3293
4. Rauch N, et al. 2022. Two-piece zirconia implants in posterior regions: a prospective cohort study with a follow-up period of 9 years.
5. Karapataki S, Fahrenholtz H, et al. Peri-implantitis and zirconia implants: results after five and up to 12 years of function. In preparation.